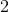Help with Rearrangement Reactions
Organic Chemistry · Learn by Concept
Help Questions
Organic Chemistry › Help with Rearrangement Reactions
Which of the following is the correct major product of the above reaction?




Explanation
Here we see an 
What is the major product of the reaction shown?


IV
I
II
III
V
Explanation
This reaction adds 


What is the major product of the reaction shown?


II
I
III
IV
None of these
Explanation
After carbocation is formed, a rearrangement reaction stabilizes positive charge by putting it on a tertiary carbon. This is done by a methyl shift. Recall that tertiary carbocations are the most stable due to the inductive effect of alkyl groups on the electron-deficient carbocation.
What is the major product of the reaction shown?
Explanation
The first step of this reaction will be protonation of the hydroxyl oxygen to create a good leaving group. When the leaving group leaves, what's left is a secondary carbocation that is vicinal to (next to) a quaternary carbon. A methyl shift is thermodynamically favored in this case, as the rearrangement will leave a tertiary carbocation. Following the rearrangement the nucleophile (bromide) will attack the tertiary carbocation, forming a sigma bond with the carbon. The answer is thus