Identifying Electron-Withdrawing Groups
Organic Chemistry · Learn by Concept
Help Questions
Organic Chemistry › Identifying Electron-Withdrawing Groups
Which of the following R groups would increase the rate of the following substitution reaction?
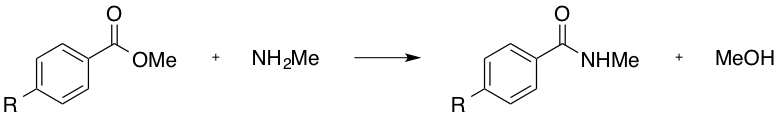
Explanation
The above reaction would more readily proceed if the electrophilicity of the carbonyl carbon were enhanced. This may be achieved through electron withdrawal via the R group.
The ether (-OMe), the methyl (-Me), and the hydroxyl (-OH), would all produce a electron-donating effect, and are thus incorrect answers.
The nitro group (-NO2), and the positively charged, tetra-substituted amino group (consider the structure once this trimethyl amino group is connected to the aryl ring) are both electron-withdrawing. As the trimethyl amino group will have an overall positive charge (and the nitro group is neutral overall), the trimethyl amino group is the stronger electron-withdrawing moiety, and is thus the correct answer.




