0%
0 / 13 answered
Thermodynamic Systems and Calorimetry Practice Test
•13 QuestionsQuestion
1 / 13
Q1
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.
$$2C_{6}$$H_{14}$(l) + $19O_{2}$(g)\rightarrow $12CO_{2}$(g) + $14H_{2}$O(l)$
 of
of  is
is  .
.
 of
of 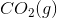 is
is .
.
 of
of 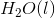 is
is  .
.
Calculate the  for the combustion of hexane liquid hexane at 298K.
for the combustion of hexane liquid hexane at 298K.
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.
$$2C_{6}$$H_{14}$(l) + $19O_{2}$(g)\rightarrow $12CO_{2}$(g) + $14H_{2}$O(l)$




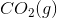


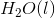

Calculate the 




 of heat energy per gram of wood consumed, what mass of wood must be consumed to heat
of heat energy per gram of wood consumed, what mass of wood must be consumed to heat  of water from
of water from  to
to  , and then to convert it to water vapor?
, and then to convert it to water vapor?